Adverse Events Following Immunization (AEFI) Statistical Data
Dates: From mid-December 2020 – April 9th, 2021
Note: Detailed AEFI Breakdowns can be found under Figure 4 (scroll down to the bottom of the page and click on the link).
⮚ COVD Vaccine Doses Administered in Canada: 7,569,321
- Previous: Feb. 12th: 1,221,539; Feb. 19th: 1,402,139; Feb. 26th:1,778,405; March 5th: 2,255,174; March 12th: 2,830,164; March 19th: 3,729,312; March 26th: 4,800,931; April 2nd: 5,897,445
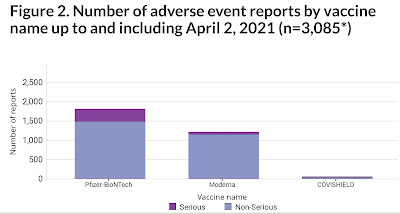
- Previous: Feb. 12th: 957; Feb. 19th: 1235; ; Feb. 26th:1591; March 5th: 1923; March 12th: 1922; ; March 19th: 2,530; March 26th: 2,859; April 2nd: 3,089
- 16 reported to not be linked to vaccine; 15 are still under investigation
- Previous: March 26th: 24 (13 not linked; 15 under investigation); April 2nd: 27 (16 reported to not be linked to vaccine; 11 are still under investigation)
- Previous: Feb. 12th: 140; Feb. 19th: 167; Feb. 26th:194; ; March 5th: 214; March 12th: 287; March 19th: 320; March 26th: 384; April 2nd: 421
- Previous: Feb. 12th: 817Feb. 19th:1068; Feb. 26th:1397; ; March 5th:1709; March 12th:1922; March 19th: 2,210; March 26th: 2,475; April 2nd: 2,668
- Previous: March 26th: 247; April 2nd:182
⮚ 45.5 reports per 100,000 doses administered (out of a total of 3,444 reports) up to and including April 9, 2021.
Definition of Serious AEFI:
An event is considered serious if it:
- results in death
- is life-threatening (an event/reaction in which the patient was at real, rather than hypothetical, risk of death at the time of the event/reaction)
- requires in-patient hospitalization or prolongation of existing hospitalization
- results in persistent or significant disability/incapacity, or
- results in a congenital anomaly/birth defect
Anaphylaxis: Among the 464 serious reports, the most frequently reported adverse event was anaphylaxis.
Special Note Regarding AstraZeneca COVID-19 vaccine: There have been reports in Europe of blood clots associated with low levels of blood platelets (thrombocytopenia) following vaccination with the AstraZeneca COVID-19 vaccine.
⮚ Following European reports of blood clots associated with low levels of blood platelets (thrombocytopenia), the National Advisory Committee on Immunization and the Chief Medical Officers of Health recently recommended that AstraZeneca COVID-19 vaccines should not be used in adults under age 55.
Source: Government of Canada. (April 20, 2021). Coronavirus Disease (COVID-19) Epidemiology Update. Retrieved from: https://health-infobase.canada.ca/covid-19/vaccine-safety/.
Canadian COVID-19 Statistics: April 20, 2021
➤ 3% of Canadians have been diagnosed with COVID-19 in 1+ year
➤️.2% Active cases in the Canadian population
➤ 90% Recovered
➤ 5% hospitalization rate
➤ ️3.9% rate of positive of 30+ million Canadians tested
➤️ .06% of Canadian population have been reported to die with COVID-19
➤ 96% of those who have died with CV-19 were over 60 years old, with pre-existing health conditions that are associated with more severe cases
Figure 3. Adverse event reports by age group up to and including April 9, 2021
Miscellaneous COVID-19 Vaccine Data
Front line Worker Testimonies: COVID-19 Vaccine Adverse Reactions. Retrieved from: https://drive.google.com/file/d/1YK0JR_lFy88Zu3rcC3L5NvL_Xr3ib6zY/view.
Copyright © 2021.Tracey Young/Canadian Advocacy Centre for Health, Safety and Justice. All Rights Reserved.
_________________________________________________
#COVID19 #COVID19Canada #COVIDvaccines #COVID19vaccines #Canada #DutytoWarn #HealthPromotion #HealthPrevention #InformedConsent #cdnpoli #canpoli








No comments:
Post a Comment